Medical Device Cleaning Validation Standards . This regulation establishes a comprehensive framework for medical. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. An overview of st98 a s medical devices become more. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. ansi/aami st98:2022 is a new, published standard. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations:
from www.orielstat.com
This regulation establishes a comprehensive framework for medical. An overview of st98 a s medical devices become more. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. ansi/aami st98:2022 is a new, published standard. new requirements for medical device cleaning validations: eu medical devices regulation (mdr) 2017/745:
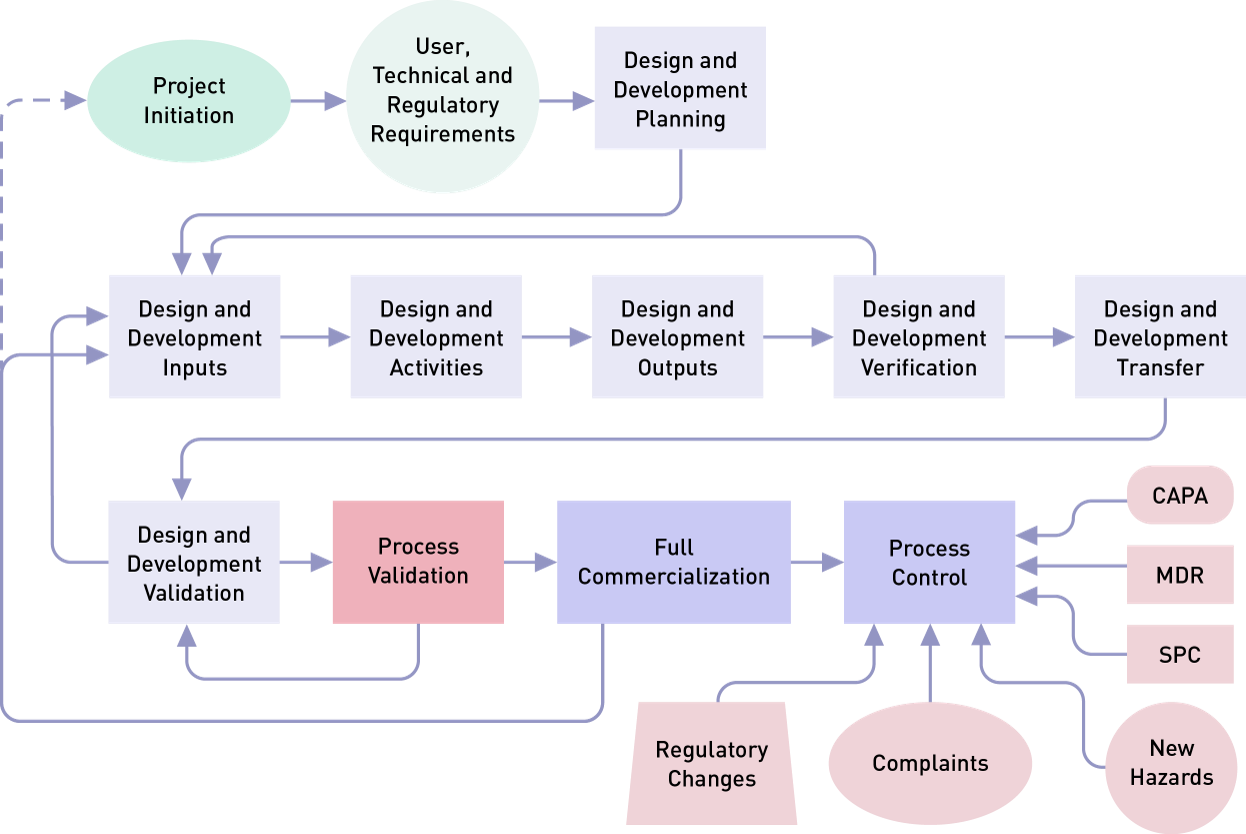
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel
Medical Device Cleaning Validation Standards This regulation establishes a comprehensive framework for medical. ansi/aami st98:2022 is a new, published standard. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations: this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. This regulation establishes a comprehensive framework for medical. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. An overview of st98 a s medical devices become more.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Cleaning Validation Standards iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. ansi/aami st98:2022 is a new, published standard. An overview of st98 a s medical devices become more. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations: 1.1 this guide provides considerations for validating. Medical Device Cleaning Validation Standards.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Cleaning Validation Standards eu medical devices regulation (mdr) 2017/745: iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. An overview of st98 a s medical devices become more. ansi/aami st98:2022 is a new, published standard. This regulation establishes a comprehensive framework for medical. this guide is designed to establish. Medical Device Cleaning Validation Standards.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Validation of Cleaning Medical Device Cleaning Validation Standards new requirements for medical device cleaning validations: ansi/aami st98:2022 is a new, published standard. eu medical devices regulation (mdr) 2017/745: It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 1.1 this guide provides considerations. Medical Device Cleaning Validation Standards.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Cleaning Validation Standards An overview of st98 a s medical devices become more. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations: ansi/aami st98:2022. Medical Device Cleaning Validation Standards.
From www.cirs-ck.com
Cleaning, Disinfection, Sterilization Process Validation Medical Medical Device Cleaning Validation Standards This regulation establishes a comprehensive framework for medical. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. ansi/aami st98:2022 is a new, published standard. eu medical devices regulation (mdr) 2017/745: An overview of st98 a s medical devices become more. this guide is designed to establish inspection consistency and uniformity by discussing. Medical Device Cleaning Validation Standards.
From www.orielstat.com
Medical Device Process Validation Plans Oriel STAT A MATRIX Medical Device Cleaning Validation Standards this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. An overview of st98 a s medical devices become more. ansi/aami st98:2022 is a new, published standard. This regulation establishes a comprehensive framework for medical. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. eu medical. Medical Device Cleaning Validation Standards.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Cleaning Validation Standards 1.1 this guide provides considerations for validating cleaning processes for medical devices during. eu medical devices regulation (mdr) 2017/745: ansi/aami st98:2022 is a new, published standard. new requirements for medical device cleaning validations: It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. An overview of st98 a s medical devices become more.. Medical Device Cleaning Validation Standards.
From templates.rjuuc.edu.np
Medical Device Verification And Validation Plan Template Medical Device Cleaning Validation Standards ansi/aami st98:2022 is a new, published standard. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. eu medical devices regulation (mdr) 2017/745: It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. This regulation establishes a comprehensive framework for medical. An overview of. Medical Device Cleaning Validation Standards.
From www.facebook.com
Tescroom Clean room Validation for Pharma, Medical Device industry Medical Device Cleaning Validation Standards this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. This regulation establishes a comprehensive framework for medical. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations: 1.1 this guide provides considerations. Medical Device Cleaning Validation Standards.
From www.presentationeze.com
QSIT Medical Device Validation requirements PresentationEZE Medical Device Cleaning Validation Standards This regulation establishes a comprehensive framework for medical. An overview of st98 a s medical devices become more. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 1.1 this guide provides. Medical Device Cleaning Validation Standards.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Medical Device Cleaning Validation Standards It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. iso 17664:2017 specifies requirements for the information to be provided by the medical device. Medical Device Cleaning Validation Standards.
From www.bioprocessonline.com
Introduction To Science And RiskBased Cleaning Validation Using ASTM Medical Device Cleaning Validation Standards 1.1 this guide provides considerations for validating cleaning processes for medical devices during. This regulation establishes a comprehensive framework for medical. new requirements for medical device cleaning validations: An overview of st98 a s medical devices become more. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. ansi/aami st98:2022 is a new, published. Medical Device Cleaning Validation Standards.
From www.presentationeze.com
Medical Device Design and Developement. Validation. Regulation. Control Medical Device Cleaning Validation Standards It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. ansi/aami st98:2022 is a new, published standard. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations: An overview of st98 a s medical devices become more. This regulation establishes a comprehensive framework for medical. iso 17664:2017 specifies requirements. Medical Device Cleaning Validation Standards.
From www.medicaldesignbriefs.com
New Requirements for Medical Device Cleaning Validations An Overview Medical Device Cleaning Validation Standards An overview of st98 a s medical devices become more. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing. Medical Device Cleaning Validation Standards.
From www.youtube.com
Key Factors that Determine a Successful Reusable Medical Device Medical Device Cleaning Validation Standards ansi/aami st98:2022 is a new, published standard. iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. This regulation establishes a comprehensive framework for medical. new requirements for medical device. Medical Device Cleaning Validation Standards.
From templates.rjuuc.edu.np
Medical Device Verification And Validation Plan Template Medical Device Cleaning Validation Standards eu medical devices regulation (mdr) 2017/745: It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. This regulation establishes a comprehensive framework for medical.. Medical Device Cleaning Validation Standards.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Cleaning Validation Standards An overview of st98 a s medical devices become more. ansi/aami st98:2022 is a new, published standard. It replaces aami tir30 and provides requirements to validate the medical device manufacturer's cleaning. new requirements for medical device cleaning validations: This regulation establishes a comprehensive framework for medical. iso 17664:2017 specifies requirements for the information to be provided by. Medical Device Cleaning Validation Standards.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device Cleaning Validation Standards iso 17664:2017 specifies requirements for the information to be provided by the medical device manufacturer for the processing of. This regulation establishes a comprehensive framework for medical. 1.1 this guide provides considerations for validating cleaning processes for medical devices during. eu medical devices regulation (mdr) 2017/745: new requirements for medical device cleaning validations: ansi/aami st98:2022 is. Medical Device Cleaning Validation Standards.